Published on April 8, 2018
(Source: PRN-Physician Recommended Nutriceuticals®)
Summary
• Diabetes Mellitus affects the pancreas which is responsible for the production of enzymes necessary to digest foods. Recent research continues to confirm that as many as 74% of Diabetic patients have a compromised ability to digest and absorb fat.
• One of the highest concentration of Omega-3s found in the body; specifically DHA, is in the retina. Peer- reviewed data is plentiful to support the nutritional need for Omega-3s EPA/DHA as a medical food to protect against the development and arrest progression of DR in the already Omega-3 fatty acids deficient diabetic patient.15i
• Individuals with type II Diabetes Mellitus without Diabetic Retinopathy should be encouraged to have an annual dilated eye examination to detect the onset of Diabetic Retinopathy.
Background
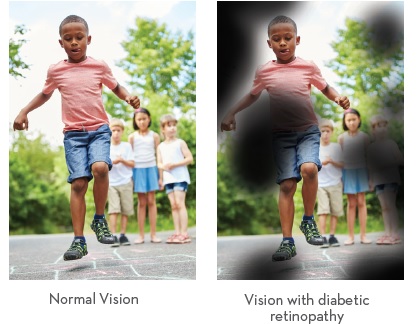
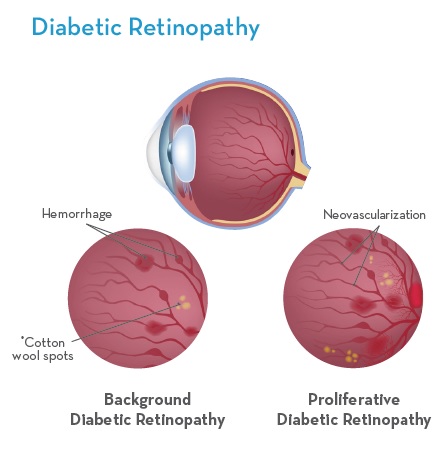
Diabetic Retinopathy is a complication of diabetes (both type I and type II). It’s caused by damage to the blood vessels of the light-sensitive tissue in the retina when high blood sugar levels are not managed.1
The market opportunity for nutritional intervention for the Diabetic population as it pertains to Diabetic Retinopathy continues to expand as the prevalence of type II Diabetes continues to rise.
Type I and Type II Diabetes
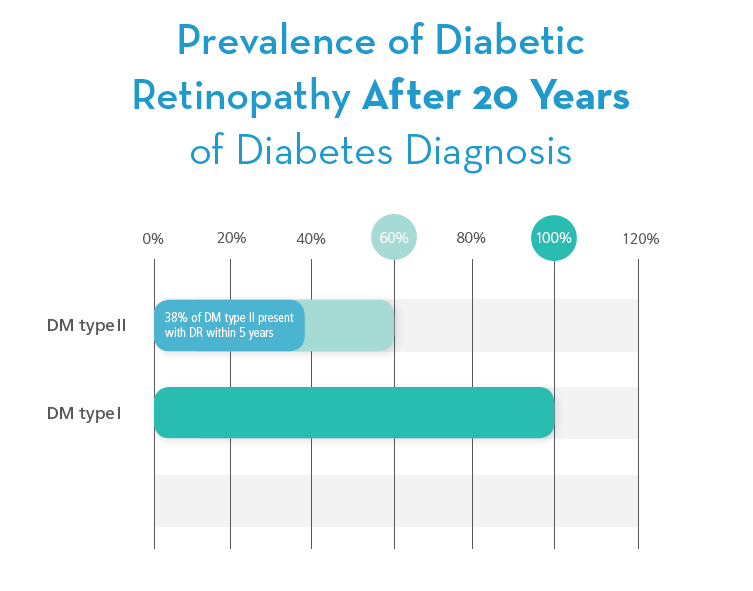
Vision-threatening retinopathy is rare in type I Diabetic patients in the first 3-5 years of Diabetes or before puberty. During the next two decades, nearly all type I Diabetic patients develop retinopathy. It is projected that 5 million people will have type I Diabetes by 2050.5 Type II Diabetes accounts for about 90% to 95% of all diagnosed cases of Diabetes. 64% of adults over 30 with type II Diabetes present with Diabetic Retinopathy in less than 5-years from diagnosis. 6
Understanding a Nutritional Imbalance of Omegas
Research has confirmed that recent dietary habits have shown a large departure from how the body is “programmed” to receive optimal nutrition by a consistent diet that provides very close to equal amounts of omega-3s and omega-6s polyunsaturated fatty acids (PUFAs).7i In the last 150 years, there has been an enormous increase in the consumption of Omega-6 fatty acids due to the increased intake of vegetable oils (corn, sunflower, safflower and soybean oils) from processed food sources.
This research also indicates that the standard Western Diet currently has a ratio of 20-30:1 Omega-6: Omega-3 instead of a more balanced ratio of 2-1:1.7ii Maintaining this balance of PUFAs is considered to be a necessary nutritional component for our general health and to maintain homeostasis within the cell membrane to turn on or off the inflammatory cascade as physiologically necessary.8 Omega-3s and Omega-6s compete for absorption; thus supplementation with specific essential fatty acids (EFAs) is necessary for individuals nutritionally compromised in addition to individuals with disease states that further compromise the body’s ability to digest and absorb fat.9
The most common Omega-3s are Alpha Linolenic Acid (ALA), Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA). ALA is the plant source Omega-3 found in green leafy vegetables and seeds like flax. ALA is the most abundant Omega-3 in nature. Unfortunately, ALA is not a preformed omega-3 fatty acid and must be biochemically converted in the body to EPA and DHA for the nutrients to be useful to offset the nutritional imbalance of Omega-3s.10
Furthermore, while ALA is used by the body as a source of energy, partly as a precursor of metabolites, the degree of conversion to beneficial Omega-3s EPA and DHA appears to be unreliable and restricted.11 EPA and DHA are known as “preformed” Omega-3s, which means that no biochemical enzymatic processes are needed for the nutrients to be immediately beneficial when consumed.12
Diabetes Mellitus affects the pancreas which is responsible for the production of enzymes necessary to digest foods. Nutritional data identifies a decrease in pancreatic function in patients with diabetes as early as 1943.13i When the role of insulin was established in the early 20th century, physicians were aware that patients with diabetes also suffered from malnutrition.13ii
The suspicion that the digestive function of the pancreas might be reduced in patients with Diabetes Mellitus prompted investigation of exocrine pancreatic function once tests became available.13iii Recent research confirms that as many as 74% of Diabetic patients have a compromised ability to digest and absorb fat further adding to the nutritional implications of an imbalance or deficiency of PUFAs.13iv Providing the most bioavailable form of EPA and DHA essential fatty acids is necessary to address this deficiency.
Diabetes Mellitus affects the pancreas which is responsible for the production of enzymes necessary to digest foods. Recent research continues to confirm that as many as 74% of Diabetic patients have a compromised ability to digest and absorb fat.
Omega-3s (EPA/DHA) and Diabetic Retinopathy
When high blood sugar levels are not managed; damage to the blood vessels of the light sensitive tissue in the retina lead to Diabetic Retinopathy. This diabetes induced injury of the retinal micro-vessels leads to the vascular pathology of Diabetic Retinopathy and ultimately vision loss.14 There are several stages of retinal vascular degeneration all of which have been shown to benefit with the introduction of Omega-3s EPA/DHA into a treatment protocol.
One of highest concentrations of Omega-3s found in the body; specifically DHA, is in the retina. Peer-reviewed data supports the nutritional need for Omega-3s EPA/DHA to protect against the development and arrest progression of Diabetic Retinopathy in the already Omega-3 fatty acids deficient diabetic patient.15i A study conducted by the National Institutes of Health (NIH) supports nutritional intervention as treatment. The researchers found that increasing Omega-3s and decreasing Omega-6 fatty acids in the diet of mice reduced the area of vessel loss that ultimately causes the growth of the abnormal vessels which may lead to blindness. Omega-6 fatty acids further contribute to the growth of abnormal blood vessels in the retina. The study showed that the mice with higher amounts of Omega-3s had a nearly 50 percent decrease in all phases of retinopathy.15ii
Early Omega-3 fatty acid intervention in Diabetic Retinopathy has also been documented reporting a DHA-rich diet fully prevented retinal vascular pathology, leading to a concomitant suppression of retinal inflammation and cellular function correction.16
Further data taken from a sub-study of the PREDIMED randomized clinical trial analyzed as an observational longitudinal cohort, considering only participants with type 2 diabetes at baseline, showed after a median follow-up of 6 years that those reporting intake of at least 500/mg daily intake of long-chain Omega-3s (EPA/DHA) at baseline had a 46% decreased risk of sight- threatening Diabetic Retinopathy compared to those not meeting the Omega-3s target intake. In addition, higher risk reductions were observed in participants with hypertension, those with diabetes of greater than 5 years duration, and those treated with insulin at baseline.17
One of the highest concentrations of Omega-3s found in the body; specifically DHA, is in the retina. Peer- reviewed data is plentiful to support the nutritional need for Omega-3s EPA/DHA as a medical food to protect against the development and arrest progression of DR in the already Omega-3 fatty acids deficient diabetic patient.15i
Currently, standard of care in late stage treatment modalities for Diabetic Retinopathy are invasive, expensive and limited to laser photo coagulation and/or intravitreal injections of vascular endothelial growth factor (VEGF) inhibitors/steroids. Late stage Diabetic Retinopathy patients can also benefit from EPA/DHA treatment as shown in a two year randomized, single-masked controlled trial. This study followed two groups of subjects with Diabetic Macular Edema (DME).18i
Group one received intravitreal ranibizumab alone; group two was given oral DHA/EPA (1,050mg/127mg day) in addition to monthly ranibizumab for four months then followed with as-needed treatments for ranibizumab.
Ranibizumab plus supplementation reduced central subfield macular thickness compared with the injections alone.18ii In addition, the supplementation group also had a significant reduction in Omega-6 arachidonic acid levels at 12 months and a significant decrease at 24 months compared with baseline.18iii Concomitantly, a similar pattern in the reduction of Omega-6 to Omega-3 ratio was observed.18iv The authors state, “the use of intravitreal ranibizumab along with DHA supplementation in patients with DME is extensively applicable to daily clinical practice”.18v
Risk Factors for Progression Once retinopathy is present, duration of diabetes appears to be a less important factor than glycemic control in forecasting progression from earlier to later stages of retinopathy.19 Intensive management of hypertension may slow retinopathy progression, yet the data remain inconclusive. Large studies have suggested that management of serum lipids may reduce retinopathy progression and the need for treatment.20 Thus, ophthalmologists should encourage patients with diabetes to be as compliant as possible with therapy of all medical and nutritional aspects of their disease.
Management of Care
Optometrists and Ophthalmologists both receive Diabetic referrals from Primary Care Physicians as well as Optometrists that only perform refraction; however, referral to an ophthalmologist is required when there is advanced non-proliferative Diabetic Retinopathy (NPDR), proliferative retinopathy (PDR), or macular edema (ME). Referral to a retinal specialist varies among the disease management in the NPDR but generally is referred before development of PDR.
The care process for Diabetic Retinopathy includes a medical history, a regular ophthalmologic examination or screening of high-quality retinal photographs of patients who have not had previous treatment for Diabetic Retinopathy or other eye disease, and regular follow-up. The purpose of an effective screening program is to determine who needs to be referred to an ophthalmologist for close follow-up and possible treatment, and who may simply be screened annually. Individuals with type II Diabetes Mellitus without Diabetic Retinopathy should be encouraged to have an annual dilated eye examination to detect the onset of Diabetic Retinopathy. Individuals with Type I Diabetes Mellitus without Diabetic Retinopathy should have annual dilated eye examinations beginning 5 years after the onset of diabetes. The recommended timing of the first ophthalmic examination and subsequent follow-up examinations for patients with diabetes varies.
Individuals with type II Diabetes Mellitus without Diabetic Retinopathy should be encouraged to have an annual dilated eye examination to detect the onset of Diabetic Retinopathy.
Patient Outcome Criteria:
• Improvement or stabilization of visual function
• Improvement or stabilization of vision-related quality of life
• Optimal control of glucose, blood pressure, and other risk factors through close communication with the patient’s primary care physician regarding the status of the Diabetic Retinopathy and the need for optimal metabolic control
References:
1. What is Diabetic Retinopathy, American Academy of Ophthalmology Kierstan Boyd, March 1, 2017 Diabetic Retinopathy, American Academy of Ophthalmology Preferred Practice Pattern, 2014.
2,5,6. CDC, 2016 Report, Diabetes.
3. Retinopathy in Diabetes; Donald S. Fong, MD, MPH, et al American Diabetes Association Diabetes Care 2004 Jan; 27(suppl 1): s84-s87.
4. WebMD Medical Reference. January 2017, American Diabetes Assoc.
7. Evolutionary aspects of diet, essential fatty acids and cardiovascular disease. Eur Heart J Supplements, Vol. 3 (Suppl D) June 2001.
8. Handbook of Diet, Nutrition and the Eye 2014. Edited by Victor R. Preedy. Chapter 17.3 Composition of skin lipids, P. viola, F. Nobili and M. Viola.
9. An Increase in the Omega-6/Omega-3 Fatty Acid Raio Increases the Risk for Obesity. Nutrients 2016, 8, 128 page 3, paragraph 1, sentence 1.
10. The Omega-3 Effect 2012, page 162.
11. Can adults adequately convert alpha-linolenic acid (18:3m-3) to eicosapentaoenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998;68(3):159-73.
12. The Omega-3 Effect 2012, page 162, paragraph 2, sentence 3.
13. Alterations in Exocrine Pancreatic Function in Diabetes mellitus. The Pancreapeia Version 1.0, February 16, 2015.
14. N-3 Polyunsaturated Fatty Acids Prevent Diabetic Retinopathy by Inhibition of Retinal Vascular Damage and Enhanced Endothelial Progenitor Cell Reparative Function PLoS One. January 2013, Vol. 8 Issue.
15. Omega-3 Fatty Acids Protect Eyes Against Retinopathy, Study Finds. National Institutes of Health(NIH) Archived Report, June 24, 2007.
16. N-3 Polyunsaturated Fatty Acids Prevent Diabetic Retinopathy by Inhibition of Retinal Vascular Damage and Enhanced Endothelial Progenitor Cell Reparative Function PLoS One. January 2013, Vol. 8 Issue.
17. Dietary Marine omega-3 fatty acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals with Type 2 Diabetes. JAMA Ophthalmol. Published online August 18, 2016.
18. Combined Intravitreal Ranibizumab and Oral Supplementation with Docosahexaenoic Acid and Antioxidants for Diabetic Macular Edema. Retina 0:1-10, 2016.
19. Klein R, Sharrett AR, Klein BE, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology 2002;109:1225-34.
20. Van Leiden HA, Dekker JM, Moll AC, et al. Blood pressure, lipids, and obesity are associated with retinopathy: The Hoorn Study. Diabetes Care 2002;25:1320-5.
21. Bioavailability of marine n-3 acid formulations, Prostaglandins Leukotrines Essent. Fatty Acids (2010), doi: 10.1016/j.plefa.2010.06.007.
